Search
Research
Impact of Parent-Reported Antibiotic Allergies on Pediatric Antimicrobial Stewardship ProgramsAntimicrobial stewardship (AMS) is crucial for optimizing antimicrobial use and restraining emergence of antimicrobial resistance. The overall increase in reported antibiotic allergies in children can pose a significant barrier to AMS, but its impact on clinical AMS care in children has not been addressed.
Research
“Our kids are our future”: Barriers and facilitators to vaccine uptake and timeliness among Aboriginal children younger than five years in Boorloo (Perth), Western AustraliaRates of several vaccine preventable diseases, and associated hospitalisation, are higher among Aboriginal and/or Torres Strait Islander children than non-Indigenous children. Western Australia has among the lowest childhood vaccine coverage in Australia, particularly among Aboriginal and/or Torres Strait Islander children. Delayed vaccination is also more common in this population. This project aimed to understand the barriers and facilitators to vaccine uptake and timeliness among Aboriginal and/or Torres Strait Islander children aged under five years in Boorloo (Perth).
Research
Management and outcomes of children hospitalised with COVID-19 including incidental and nosocomial infections in Australia 2020–2023: A national surveillance studyManagement and outcomes of children hospitalised with acute SARS-CoV-2 infection may differ throughout the pandemic or with admission type (clinical COVID-19, incidental COVID-19 or nosocomial infection).
Research
Nasal Delivery of Haemophilus haemolyticus Is Safe, Reduces Influenza Severity, and Prevents Development of Otitis Media in MiceDespite vaccination, influenza and otitis media (OM) remain leading causes of illness. We previously found that the human respiratory commensal Haemophilus haemolyticus prevents bacterial infection in vitro and that the related murine commensal Muribacter muris delays OM development in mice. The observation that M muris pretreatment reduced lung influenza titer and inflammation suggests that these bacteria could be exploited for protection against influenza/OM.

News & Events
The Kids Research Institute Australia launches Covid-19 booster research to inform Australia’s vaccine policyOptimising our national Covid-19 vaccine program could be one step closer thanks to new research now underway at The Kids Research Institute Australia investigating the most effective, long-term strategies for booster vaccinations.

News & Events
Latest Deborah Lehmann Research Award recipient tackles malaria in MadangPapua New Guinean researcher Dr Lincoln Timinao has been awarded the 2025 Deborah Lehmann Research Award (DLRA) for his work aimed at investigating the burden of malaria in young children.
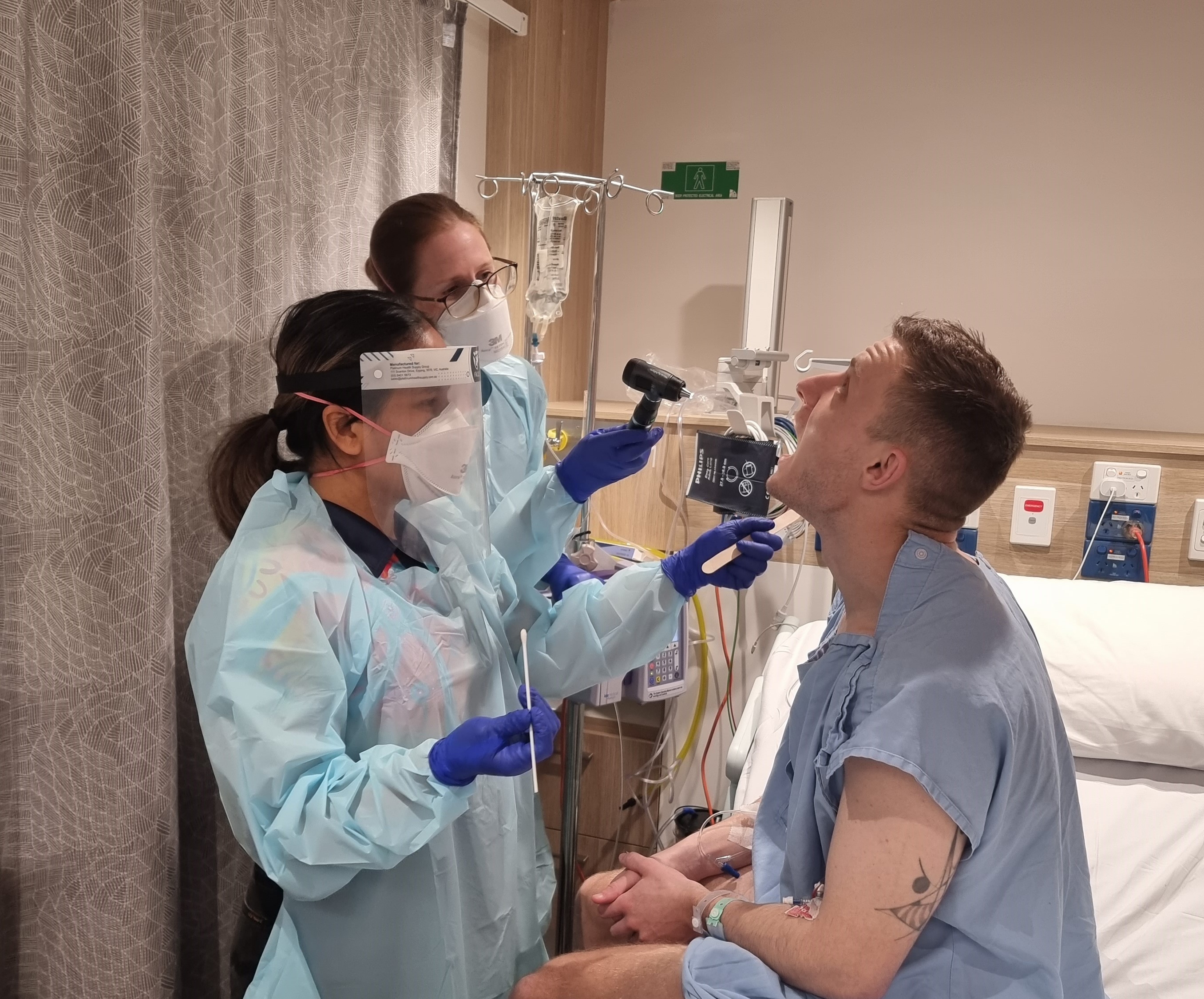
News & Events
Study which deliberately infected participants leads to penicillin breakthroughA unique study purposely giving participants Streptococcus pyogenes (Strep A) to learn how much penicillin it takes to prevent infection has found the amount needed is much lower than previously thought – a discovery that will transform thinking on treatment for people living with rheumatic heart disease (RHD).
Conference presentations in 2025

Register now and experience an awesome adventure into the world of Science, Technology, Engineering and Mathematics this August!
