Search
Showing results for "Au"
News & Events
Getting through to young people on sun safety and vapingResearchers from The Kids Research Institute Australia and Cancer Council WA will team up on two projects aimed at identifying the most effective public health messaging for young people around SunSmart behaviours and how to stop vaping.

News & Events
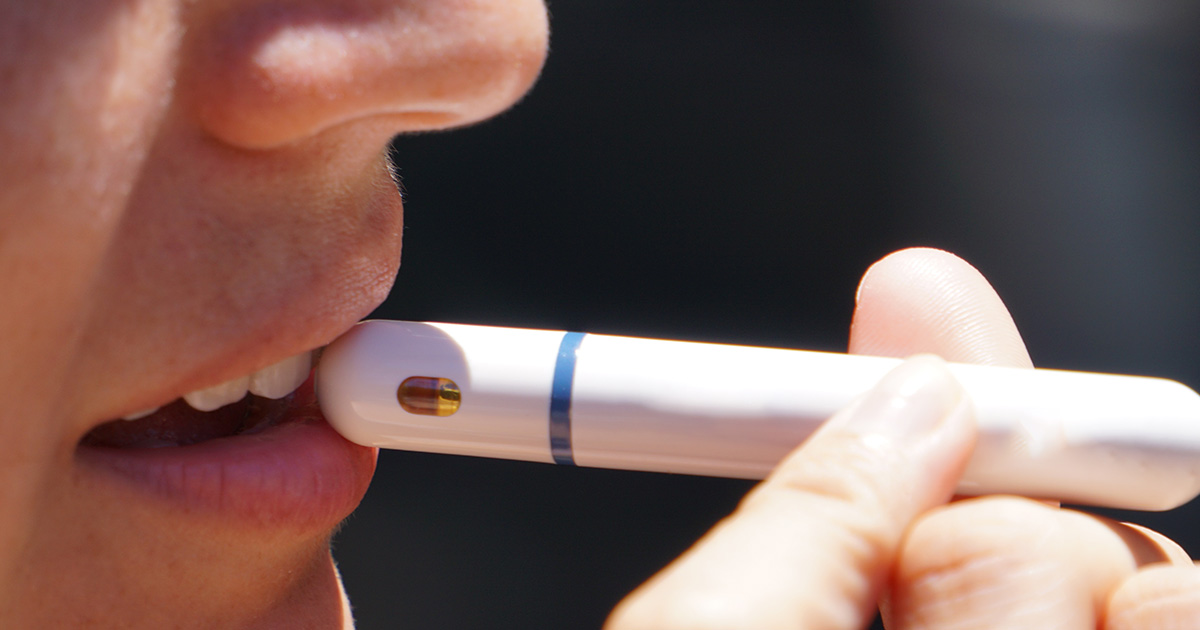
Toxic, harmful chemicals found in popular Australian e-liquidsPerth researchers have found toxic and harmful chemicals in several dozen e-cigarette liquids readily available in Australia.

News & Events
Antiviral drug shown to speed up COVID-19 recoveryAn international research collaboration, including The Kids Research Institute Australia infectious disease specialist Tobias Kollmann, has shown that the antiviral drug Interferon can speed up the recovery of COVID-19 patients.
Research
Antecedents of hospital admission for deliberate self-harm from a 14-year follow-up study using data-linkageA prior episode of deliberate self-harm (DSH) is one of the strongest predictors of future completed suicide. Identifying antecedents of DSH may inform strategi
Research
Antecedents of teenage pregnancy from a 14-year follow-up study using data linkageThis study identified possible antecedents of teenage pregnancy using linked data from administrative sources to create a 14-year follow-up from a cross-sect...
Research
Qualitative assessment of healthy volunteer experience receiving subcutaneous infusions of high-dose benzathine penicillin G (SCIP) provides insights into design of late phase clinical studiesSecondary prophylaxis to prevent rheumatic heart disease (RHD) progression, in the form of four-weekly intramuscular benzathine benzylpenicillin G (BPG) injections, has remained unchanged since 1955. Qualitative investigations into patient preference have highlighted the need for long-acting penicillins to be delivered less frequently, ideally with reduced pain.
Research
A Systematic Framework for Prioritizing Burden of Disease Data Required for Vaccine Development and Implementation: The Case for Group A Streptococcal DiseasesVaccine development and implementation decisions need to be guided by accurate and robust burden of disease data. We developed an innovative systematic framework outlining the properties of such data that are needed to advance vaccine development and evaluation, and prioritize research and surveillance activities.
Research
Lack of effectiveness of 13-valent pneumococcal conjugate vaccination against pneumococcal carriage density in Papua New Guinean infantsPapua New Guinea (PNG) introduced the 13-valent pneumococcal conjugate vaccine (PCV13) in 2014, with administration at 1, 2, and 3 months of age. PCV13 has reduced or eliminated carriage of vaccine types in populations with low pneumococcal carriage prevalence, carriage density and serotype diversity.
Research
Examining the entire delayed respiratory syncytial virus season in Western AustraliaAn interseasonal resurgence of respiratory syncytial virus (RSV) was observed in Western Australia at the end of 2020. Our previous report describing this resurgence compared the 2019 and 2020 calendar years, capturing only part of the 2020/21 season.
Research
Study protocol for controlled human infection for penicillin G against Streptococcus pyogenes: a double-blinded, placebo-controlled, randomised trial to determine the minimum concentration required to prevent experimental pharyngitis (the CHIPS trial)Regular intramuscular benzathine penicillin G injections have been the cornerstone of rheumatic heart disease (RHD) secondary prophylaxis since the 1950s. As the pharmacological correlate of protection remains unknown, it is difficult to recommend changes to this established regimen. Determining the minimum effective penicillin exposure required to prevent Streptococcus pyogenes infection will accelerate development of new long-acting penicillins for RHD prevention as well as inform opportunities to improve existing regimens. The CHIPS trial will address this knowledge gap by directly testing protection afforded by different steady state plasma concentrations of penicillin in an established model of experimental human S. pyogenes pharyngitis.
